Olympus 3D/FlexDex® for Minimal Access Surgery Simplifies Suturing, Redefines Robotics
Powerful Pairing of ENDOEYE FLEX 3D and FlexDex Needle Driver to Be Demonstrated at American College of Surgeons Clinical Conference
The Olympus 3D/FlexDex platform offers an alternative to high-cost robotics in minimal access surgery by providing the visualization and wristed instrumentation for suturing found in robotic technology, but at a fraction of the cost. 3D/FlexDex can be used during many types of procedures, including but not limited to general surgery and gynecological specialties, and will be on display at the American College of Surgeons Clinical Conference, October 21-25, 2018, in Boston.
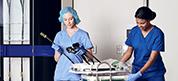
CENTER VALLEY, Pa., (October 18, 2018) – Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, among other core businesses, announced today the launch of the ENDOEYE FLEX 3D and the FlexDex® Needle Driver (3D/FlexDex®) laparoscopic surgical solution. Through an exclusive U.S. distribution agreement, Olympus is leveraging its innovative ENDOEYE FLEX 3D laparoscopic imaging technology with the wristed needle-driver of FlexDex to enhance the capabilities of minimal access surgery while providing a cost-effective robotic solution. 3D/FlexDex can be used during many types of procedures, including but not limited to general surgery and gynecological specialties.
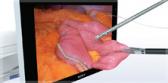
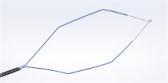
Olympus’ 3D imaging combined with FlexDex technology simplifies suturing in difficult-to-reach areas by precisely translating the surgeon’s hand, wrist and arm movements from outside the patient into corresponding movements of an end-effector inside the patient’s body. With a chip-on-tip and dual lens design, the ENDOEYE FLEX 3D video laparoscope restores natural 3D vision and depth perception during laparoscopic procedures and mimics what the human eye would see in open surgery. The 3D/FlexDex platform offers an alternative to high-cost robotics in minimal access surgery by providing the visualization and wristed instrumentation for suturing found in robotic technology, but at a fraction of the cost. Importantly, this technology can be used in any OR, at any time, on a laparoscopic platform already familiar to surgeons. With Olympus’ extensive market reach and advanced 3D technology, 3D/FlexDex is poised to redefine robotics in minimal access surgery.
Features of 3D/FlexDex include:
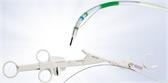
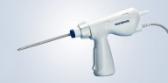
- Three-axis gimbal that attaches to a surgeon’s wrist and, once attached, leverages a series of mechanical components to translate the movement of the surgeon’s hand to the tip of the instrument
- Purely mechanical, low cost and disposable instrument that provides robotic functionality with laparoscopic tactile feedback
- Observation and therapy enabled in the entire peritoneal cavity by maintaining optimum and correct visual orientation with up to 100º of articulation in all directions, and utilizing high density CCD image sensors to provide dynamic, high-definition 3D images
“I am very excited about the 3D/FlexDex platform. My initial findings based on performing over 200 surgeries using the platform indicate significant improvement in suturing technique, with an elimination of all tacking devices and all surgeries completed without complications,” said Dr. Kent Bowden, DO, FACOS. “Surgeons such as myself serving a rural population of patients in small community hospitals need the advanced capabilities and cost-effective solutions provided by the 3D/FlexDex platform.”
“Olympus and FlexDex are redefining minimal access surgery in the OR,” said Todd Usen, President, Olympus Medical Systems Group. “We expect this solution to offer surgeons the precision and control they desire without the burden of a complex and costly computer-aided robotic system. This will not only improve patient outcomes and satisfaction but also help healthcare institutions provide true value-based care.”
“Through our Olympus partnership, FlexDex is going to disrupt the paradigm where surgeons and hospitals had to choose between high cost/high function and low cost/low function,” said Dr. James Geiger, CEO at FlexDex.
3D/FlexDex will be launched to the healthcare community at the American College of Surgeons Clinical Conference, October 21-25, 2018, in Boston.
To learn more about 3D/FlexDex, please call 1-800-848-9024 or visit us at http://medical.olympusamerica.com.
# # #
About Olympus Medical Systems Group
Olympus is a global technology leader, crafting innovative optical and digital solutions in medical technologies; life sciences; industrial solutions; and cameras and audio products. Throughout our nearly 100-year history, Olympus has focused on being true to society and making people’s lives healthier, safer and more fulfilling.
Our Medical Business works with health care professionals to combine our innovative capabilities in medical technology, therapeutic intervention, and precision manufacturing with their skills to deliver diagnostic, therapeutic and minimally invasive procedures to improve clinical outcomes, reduce overall costs and enhance quality of life for patients. For more information, visit medical.olympusamerica.com.
About FlexDex
FlexDex is an innovative medical device company that has developed a platform technology to provide surgeons with high performance and cost effective minimally invasive surgical instruments. The Company’s focus is to enable advanced minimally invasive procedures with greater precision and efficiency bringing the benefits of minimally invasive surgery to patients throughout the world. FlexDex was developed at the University of Michigan by co-founders Shorya Awtar, Sc.D., James Geiger, MD and Greg Bowles. This platform technology enables highly intuitive, one-to-one mapping of the surgeon’s arm and hand motions to the articulating instrument inside the patient’s body. The patented “Virtual Center™” of the FlexDex platform is a simple mechanical design that, we believe, will greatly enhance the capabilities of all Minimally Invasive Surgical (MIS) instruments. The FlexDex Needle Driver, the first instrument of the FlexDex platform, has been granted the CE mark and is available for sale in the United States and internationally. For more information visit https://flexdex.com.