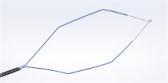
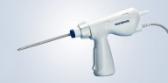
Olympus Adds SmartBand® Multi-Band Ligation Kit to EndoTherapy Portfolio through Exclusive Distribution Agreement
The first scientifically designed anti-slip, latex-free band delivers life-saving treatment for endoscopic variceal ligation

Olympus announced today its exclusive distribution agreement with Intelligent Endoscopy. Beginning immediately, SmartBand® Multi-Band Ligation Kit, cleared by the FDA to endoscopically ligate esophageal varices at or above the gastroesophageal junction and internal hemorrhoids, will be distributed by Olympus America Inc. (OAI). SmartBand offers the first scientifically designed anti-slip, latex-free bands to provide needed gripping force to treat esophageal varices, an often deadly condition associated with cirrhosis of the liver, a disease that afflicts 3.9 million in the United States.
CENTER VALLEY, Pa., (January 29, 2018) – Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, among other core businesses, announced today its exclusive distribution agreement with Intelligent Endoscopy. Beginning immediately, SmartBand® Multi-Band Ligation Kit, cleared by the FDA to endoscopically ligate esophageal varices at or above the gastroesophageal junction and internal hemorrhoids, will be distributed by Olympus America Inc. (OAI).
SmartBand offers the first scientifically designed anti-slip,latex-free band intended to stay on the tissue for more reliable band retention. Historically, latex-free bands lacked the elasticity or gripping force to provide sufficient band retention. According to the Manufacturer and Usability Database Experience (MAUDE) maintained by the FDA, band slippage is one of the highest reported complaints with endoscopic band ligators.
SmartBand is designed to improve ligation performance through its:
- Anti-slip band design: SafeGrip’s proprietary anti-slip structures grip and trap the tissue within the recessed grooves. The result is a 33% improvement in band gripping force, keeping the band on the tissue to stop the bleeding.i
- Predictable band deployment: The unique handle, designed using human factors, is engineered to reliably predict band deployment so with a natural twist of the wrist, the operator is able to release each band with confidence.
- Easy, Intuitive Setup: An intuitive hook and loop theme helps to load the device correctly, the first time.
- Replacement Packs: Decrease procedure time and costs by eliminating the need to open a new kit when more bands are needed. .
Esophageal varices, a sometimes deadly condition associated with cirrhosis of the liver, is a condition afflicting 3.9 million people in the United States and resulting in more than 38,000 deaths each year, according to the Centers for Disease Control. Esophageal varices result from slower liver processing of blood, which causes portal hypertension that backs the blood into the esophageal veins, causing them to bulge until they sometimes burst. Endoscopic variceal ligation (EVL) is a procedure whereby bands are deployed to close off the blood flow to the engorged veins, forcing the body to produce new tissue in the esophagus. The most recent American Association for the Study of Liver Disease (AASLD) and Baveno V consensus guidelines suggest that all patients who have been diagnosed with cirrhosis undergo screening endoscopy to assess for esophageal and gastric varices.ii , iii However, most people do not know they have esophageal varices until the varices start to bleed, at which point rapid medical intervention and excellent tools are needed.
EVL has been considered the gold standard for controlled esophageal varices since the mid 1990s. A meta-analysis has confirmed the superiority of EVL compared with the leading alternative treatment for all major outcomes, including survival (recurrent bleeding, local adverse events including ulceration and stricture formation, time to variceal obliteration, and survival).iv Furthermore, consensus guidelines recommend repeat endoscopy with EVL every 1 to 2 weeks until obliteration is observed. iii
“This distribution agreement with Intelligent Endoscopy complements our robust portfolio for endoscopic treatment of major GI conditions,” Kurt Heine Group Vice President, Endoscopy Division at Olympus America Inc. “Our focus on finding the best endoscopic devices bolsters our already leading role in visualization and processing solutions for the GI team. These synergies can help contribute to better quality care, cost reduction and improved patient satisfaction.”
“We are pleased that this distribution agreement will bring SmartBand to an ever-wider base of physician users,” said Fritz Haller, Managing Director, Intelligent Endoscopy. “Olympus is a leader in equipment and procedures for treating GI diseases, and is the logical distribution choice.”
To learn more about SmartBand, please call 1-800-848-9024 or visit us at http://medical.olympusamerica.com.
# # #
About Olympus Medical Systems Group
Olympus Medical Systems Group, a division of global technology leader Olympus, develops solutions for healthcare professionals that help improve clinical outcomes, reduce overall costs and enhance quality of life for their patients. By enabling less invasive procedures, innovative diagnostic and therapeutic endoscopy, and early stage lung cancer evaluation and treatments, Olympus is transforming the future of healthcare. For more information visit Olympus at www.medical.olympusamerica.com.
i Data on file at Intelligent Endoscopy.
ii Garcia-Tsao G, Sanyal AJ, Grace ND, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007;46:922-38.
iii de Franchis R. Revising consensus in portal hypertension: report fo the Baven V consensus workshop on metholdology of diagnosis and therapy in portal hypertension. J Hepatol 2010;53:762-8.
iv Laine L, Cook D. Endoscopic ligation compared with sclerotherapy for treatment of variceal bleeding. A meta-analysis. Ann Intern Med. 1995;123(4):280-287.