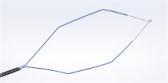
IBV Valve System
The IBV Valve System is a minimally invasive treatment designed to control acute and chronic conditions of the lung. During surgery, these small umbrella-shaped Olympus valves are deployed into the airways of the lungs via a catheter passed through a bronchoscope; once in place, the IBV Valves temporarily prevent air from flowing distally to the injured tissue while still allowing mucus to exit through normal ciliary transport. The valves are later removed via a similar bronchoscopic procedure.
Caution: Humanitarian Device. Authorized by federal law for the control of prolonged air leaks of the lung, or significant air leaks that are likely to become prolonged, following lobectomy, segmentectomy, or LVRS (lung volume reduction surgery). The effectiveness of this device for this use has not been demonstrated. Federal law restricts this device to sale by or on the order of a physician. The IBV Valve is not yet approved for use in emphysema, but is subject to an ongoing clinical investigation in which approval for use in emphysema will be sought. For more information, visit www.ibvinstructions.com or contact a sales representative at 866-497-1700, ext. 200.