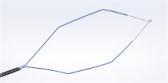
Olympus Expands Voluntary Recall for ViziShot 2 FLEX (19G) EBUS -TBNA Needles
CENTER VALLEY, Pa., (January 16, 2026) – Olympus Corporation has announced the expansion of a previous global medical device removal action for ViziShot 2 FLEX (19G) EBUS -TBNA needles (“ViziShot 2 FLEX”) after receiving and investigating complaints of device components ejecting or detaching during procedures. The complaints included adverse event reports of patient injury and one death. This expanded action includes all lots of the ViziShot 2 FLEX needles and supersedes the August 2025 notice in which only certain lots of the device were being recalled.
Potential consequences of a detached component of the ViziShot 2 FLEX include the risk of unintended device components within the tracheobronchial tree that may require bronchoscopic extraction or surgical removal.
Since the August 2025 notice, internal investigations of complaints identified additional contributing factors to hypotube component ejection including device heat-shrink material degradation and use errors, which affect all lots of the ViziShot 2 FLEX device. The device heat-shrink material that seals the needle can degrade during clinical use and may result in difficulty in extracting or expelling samples, fluid leakage, impaired needle deployment or retraction, or breakage of device components.
Users are directed to cease use immediately and quarantine all ViziShot 2 FLEX devices. Customers have been instructed to return the affected devices to Olympus by following the instructions provided in the removal action communication. Patient safety is our top priority.
The ViziShot 2 FLEX is designed to be used with ultrasound endoscopes for ultrasound guided fine needle aspiration and fine needle biopsy of submucosal and extramural lesions of the tracheobronchial tree.
Other EBUS-TBNA needles are not affected by this action.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch program online: https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program/reporting-serious-problems-fda
For information on how to identify affected product, U.S. customers can contact the Olympus Technical Assistance Center (TAC) at 1-800-848-9024, Option 1, Monday-Friday between 7:00 AM and 8:00 PM ET. U.S. customers can report a problem with the device to TAC by phone, or by email at complaints@olympus.com.