Olympus Launches HANAROSTENT Self-expanding Metal Stents to EndoTherapy Portfolio at DDW
New LowAx™ Stents Allow Palliative Treatment of Colonic and Duodenal Obstructions
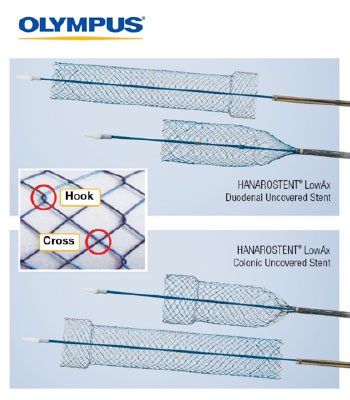
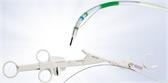
HANAROSTENT® LowAx™ Colonic and Duodenal Uncovered Stents are 510(K) cleared devices made by M.I. Tech and now distributed exclusively through Olympus in the U.S. Both are used for the treatment of strictures to improve quality of life.
CENTER VALLEY, Pa., (May 14, 2019) – Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, among other core businesses, announced the launch of two additional HANAROSTENT® self-expanding metal stents (SEMS) at Digestive Disease Week (DDW) 2019. These new additions to the Olympus EndoTherapy portfolio for advanced procedures include the 510(K) cleared HANAROSTENT LowAx™ Colonic and Duodenal Uncovered Stents made by M.I. Tech and now distributed exclusively through Olympus in the U.S.
In the June 2019 issue of Gastroenterology & Endoscopy News (expected publication date June 15, 2019), Drs. Sherif Andrawes and David Diehl describe the SEMS as an “attractive alternative to surgery,” and specify cases for use as a palliative measure for patients with short life expectancy or those with malignant colonic or duodenal obstruction. The SEMS can be placed easily under fluoroscopic and endoscopic guidance to restore patency with a design that minimizes the risk of migration. Patients benefit from SEMS placement as a palliative measure that enables rapid reversal of symptoms such as nausea, vomiting, abdominal discomfort, and weight loss, without the risks associated with an invasive surgical procedure. The doctors further specify that colonic SEMS placement “also can serve as a bridge to surgery for patients with an acute colon obstruction, providing necessary bowel decompression until elective 1-stage surgery can be performed.”
These colonic and duodenal SEMS will bring further clinical differentiation to the existing luminal patency market. Both stents provide:
- Clinical Value: Hook-cross braided design allows for optimal radial/axial force and segmental compression; designed to maximize patient comfort and quality of life.
- Economic Value: Conforming design adapts to anatomy to maintain patency, reduces the risk of occlusion and ultimately minimizes re-intervention costs.
- Efficiency: Flexible catheter design for tight stricture navigation, smooth deployment and overall ease of use.
“Palliation of symptoms through the new HANAROSTENT for colonic and duodenal obstructions can help improve the quality of life for patients facing a malignant diagnosis or obstruction,” said Kurt Heine, Group Vice President of the Endoscopy Division at Olympus America Inc. “Olympus is pleased to continue expanding the line of these unparalleled stents to physicians and patients in the U.S.”
The HANAROSTENT Colonic (25mm, 22mm) and Duodenal (22mm) SEMS are available in 3 lengths to target different strictures; 6cm, 9cm and 12cm.
Dr. Harshit Khara will be discussing advances in luminal stenting at Olympus Booth #1443 on Monday, May 20, 2019 at 12:00 PM at DDW in San Diego.
# # #
About Olympus Medical Systems Group
Olympus is a global technology leader, crafting innovative optical and digital solutions in medical technologies; life sciences; industrial solutions; and cameras and audio products. Throughout our nearly 100-year history, Olympus has focused on being true to society and making people’s lives healthier, safer and more fulfilling.
Our Medical Business works with health care professionals to combine our innovative capabilities in medical technology, therapeutic intervention, and precision manufacturing with their skills to deliver diagnostic, therapeutic and minimally invasive procedures to improve clinical outcomes, reduce overall costs and enhance quality of life for patients. For more information, visit medical.olympusamerica.com.