Olympus Launches Single-Use Procedure Kits and Hybrid Tubing
New Procedure Kits and Hybrid Tubing Streamline Procedures and Facilitate Post-Procedure Pre-Cleaning
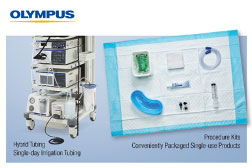
Olympus introduces Procedure Kits and Irrigation Tubing, solidifying its position as a total solutions provider in Endoscopy.
CENTER VALLEY, Pa., (November 4, 2020) – Olympus announces today the launch of the Olympus Procedure Kits, in partnership with Ruhof Corporation, and Hybrid Tubing, two new single-use and single-day devices that reduce the chances for cross-contamination.
“The introduction of the Olympus Procedure Kits and Hybrid Tubing further demonstrates our commitment to infection prevention,” said Melinda Benedict, MS, CIC, CFER, Senior Manager of Infection Prevention at Olympus. “This launch represents an important step forward in ensuring that we meet customer needs by helping them to complete their reprocessing procedures safely, efficiently, and in compliance with manufacturer instructions for use and FDA guidelines.”
Procedure Kits
Solidifying its position as a total solutions provider for Endoscopy, Olympus has partnered with Ruhof Corporation to bring Procedure Kits into its growing EndoTherapy portfolio. The kits, also known as compliance kits, convenience kits, or bedside kits, are a convenient grouping of products designed to aid in the setup and execution of applicable procedures and improve procedural efficiency and post-procedure pre-cleaning. Common items found in the kits include Air/Water, Suction, & Biopsy Valves, Gauze Pads, Lubricant, Chux/Underpads, Suction Tubing, Emesis Basins, Enzymatic Sponges, Flushing Syringes, and more.
Professional societies are supporting research demonstrating that proper reprocessing of endoscopes and accessories is imperative to the safe and effective treatment of patients.1,2 Adoption of procedure kits enables customers to properly care for their endoscopes and reduce the risk of infection. All components are focused on finding efficiencies within three areas:
- Preventing Infection:
- Kit components are single-use, eliminating the potential for reuse or cross-contamination
- ECO-Bedside Kit, enhanced with Bio-Clean technology, begins cleaning on contact
- Number of contact points during transportation compared to stocking individual items is significantly reduced
- Improving Procedural Efficiency:
- Efficiency is increased before, during, and after the procedure by ensuring that all cleaning and procedural items are available
- Procedure kits reduce ordering time, inventory management costs, and packaging waste
- Guardian Valves are the only single-use valves validated by Olympus for use with Olympus scopes and eliminate the burden of manual cleaning and reprocessing
- Enabling Proper Care:
- Procedure kits ensure that all necessary items are available at point-of-use
- Guidelines for processing and transportation are met, helping to reduce the potential for endoscope damage
- Consistent quality of care through standardization in every case and in the reprocessing cycle
“We are thrilled to partner with Olympus to offer reliable solutions that meet the regulations and standards of U.S. customers,” said Douglas Mackay, Vice President of Sales and Marketing at Ruhof Corporation. “The powerful combination of our highly advanced product line and Olympus’ leadership in endoscopy is pivotal to addressing the ever-growing concern around cross contamination in hospitals.”
Hybrid Tubing
The latest addition to the Olympus tubing portfolio is the Hybrid Tubing. This combination tubing allows for input of air or CO2 and water through the scope and jet function. The all-in-one design is helping to decrease procedural setup time and increase efficiencies in the endoscopy suite. Similar to the Procedure Kits, the single-day tubing eliminates the need for the reprocessing of reusable tubing.
'Additional features of the new Hybrid Tubing include:
- Efficiency:
- Single-day tubing eliminates the need for reprocessing of reusable tubing, minimizing infection prevention risks
- Tube markers make identification of pump head position on the irrigation tube more intuitive and visually obvious
- The all-in-one tubing design helps to decrease procedural setup time
- Strong Seals, Strong Performance:
- Two-piece cap features tubing that is bonded to the inner cap, ensuring a strong seal
- Two-piece cap seals without the need for a separate part, ensuring optimal sealing to disposable water bottles
- Dual-Port connector features a unique tunnel connection, helping to maintain shape and sealing ability over the course of multiple scope changes
- Ease of Use:
- Two-Piece Cap designed for single-handed attachment to the bottle without twisting the tubes
- Pinch clamp designed in a simple on/off design, ensuring flow of water is prevented when engaged
- Dual Port connector features an ergonomic grip for easy identification and simplified connection
For more information on Procedure Kits, Hybrid Tubing, or Olympus’ growing EndoTherapy portfolio, visit https://medical.olympusamerica.com/products/procedure-kits or https://medical.olympusamerica.com/endotherapy.
# # #
About Olympus
Olympus is passionate about the solutions it creates for the medical, life sciences, and industrial equipment industries, as well as cameras and audio products.
Olympus’ Medical business uses innovative capabilities in medical technology, therapeutic intervention, and precision manufacturing to help healthcare professionals deliver diagnostic, therapeutic, and minimally invasive procedures to improve clinical outcomes, reduce overall costs, and enhance the quality of life for patients. Olympus’ Medical portfolio includes endoscopes, laparoscopes, and video imaging systems, as well as surgical energy devices, system integration solutions, medical services, and a wide range of endotherapy instruments. For more information, visit medical.olympusamerica.com.
1 Yong E, Zenkova O, Sabil F, Cohen LB, Rhodes K, Rabeneck L. Efficiency of an endoscopy suite in a teaching hospital: delays, prolonged procedures, and hospital waiting times. Gastrointestinal Endoscopy. https://www.giejournal.org/article/S0016-5107(06)00440-8/pdf. Published November 1, 2006.
2 Standards of Infection Prevention in Reprocessing Flexible Gastrointestinal Endoscopes. SGNA. https://www.sgna.org/Portals/0/SGNA%20Standards%20of%20infection%20prevention%20in%20reprocessing_FINAL.pdf?ver=2018-11-16-084835-387. Published 2018.