Olympus Subsidiary Arc Medical Settles Patent Infringement Case, Medivators to Cease Sale of AmplifEYE

CENTER VALLEY, Pa. (December 15, 2021) — Olympus Corporation and Medivators announced today the termination of the pending patent infringement action brought by Olympus in March 2021 regarding the AmplifEYE™ products. The settlement resolves the pending action before the Texas court alleging Medivators’ infringement of two of Arc Medical’s patents. Medivators has announced it will cease the sale or distribution of AmplifEYE products after December 31, 2021. The specific terms of the agreement remain confidential. Arc Medical is a subsidiary of Olympus Corporation.
“We are pleased with the terms of this settlement as it demonstrates Olympus Corporation’s commitment to invest in and defend its intellectual property throughout the world,” said Gael Tisack, Global Head of Intellectual Property for Olympus Corporation.
About ENDOCUFF
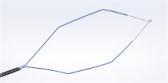
The patented ENDOCUFF™ device, invented by Arc Medical, is an assistive technology for colonoscopy that has been shown to increase the rate of adenoma detection by as much as 11 percent compared to standard colonoscopy.i Research shows that, for every 1% increase in ADR, there is a 3% reduction in the risk of interval colorectal cancer and a 5% reduction in the risk of fatal colorectal cancer (CRC).ii Higher detection rates and more accurate diagnosis through screening can reduce the number of deaths from preventable digestive cancers, such as colorectal cancer, which is a leading cause of cancer death for both men and women worldwide.iii
Attaching to the distal end of a colonoscope, the flexible ENDOCUFF arms smooth the large folds of the mucosa lining the colon, allowing the physician to inspect more of the mucosal surface area for abnormalities. The action of the ENDOCUFF arms also anchor the scope tip during loop reduction and stabilize the scope tip during complex procedures, such as a polypectomy.
In addition, a recently published meta-analysis of randomized controlled trials concludes that consistent use of the ENDOCUFF VISION™ device was associated with a significant improvement in adenoma detection rate, adenomas per colonoscopy, and a reduction in the mean withdrawal time without any increase in adverse events compared to unassisted colonoscopy.iv
# # #
About Olympus
As a leading medical technology company, Olympus uses innovative capabilities in medical technology, therapeutic intervention, and precision manufacturing to help healthcare professionals deliver diagnostic, therapeutic, and minimally invasive procedures to improve clinical outcomes, reduce overall costs, and enhance the quality of life for patients. Olympus’ Medical portfolio includes endoscopes, laparoscopes, and video imaging systems, as well as surgical energy devices, system integration solutions, medical services, and a wide range of EndoTherapy devices. For more information, visit www.medical.olympusamerica.com.
Media Contacts:
Jennifer Bannan
Olympus Corporation of the Americas
Director, PR and Medical Communications
(412) 403-8742
Jennifer.bannan@olympus.com
- Williet N, Tournier Q, et al. Effect of Endocuff-assisted colonoscopy on adenoma detection rate: meta-analysis of randomized controlled trials. Endoscopy. 2018;50(9):846-860. doi: 10.1055/a-0577-3500.
- Corley DA, Jensen CD, Marks AR, et al. Adenoma Detection Rate and Risk of Colorectal Cancer and Death. N Engl J Med. 2014;370:1298-1306. doi: 10.1056/NEJMoa1309086.
- American Cancer Society. Key statistics for colorectal cancer. https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html. Accessed December 10, 2021.
- Patel HK, Chadrasekar VT, Srinivasan S, et al. Second-generation distal attachment cuff improves adenoma detection rate: meta-analysis of randomized controlled trials. Gastrointest Endosc. 2020;93(3):544-553.e7. doi: 10.1016/j.gie.2020.09.045.