Olympus Wins Silver in 2019 Edison Awards
3D/FlexDex® recognized as innovative laparoscopic surgical solution
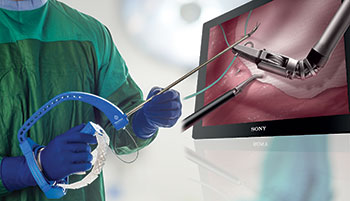
ENDOEYE FLEX 3D & FlexDex Surgical Solution
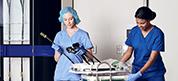
CENTER VALLEY, Pa., (April 8, 2019) – Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, among other core businesses, won Silver in the Medical category of the 2019 Edison Awards™ for the ENDOEYE FLEX 3D and FlexDex Needle Driver, developed by FlexDex, Inc. (3D/FlexDex). The distinguished awards, inspired by Thomas Edison's persistence and inventiveness, recognize innovation, creativity and ingenuity in the global economy.
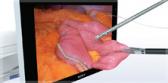
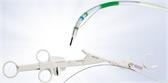
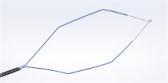
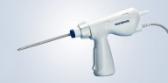
Olympus’ 3D imaging combined with FlexDex technology simplifies suturing in difficult-to-reach areas. The ENDOEYE FLEX 3D video laparoscope enables the natural 3D vision and depth perception of open surgery, while the FlexDex wristed needle driver precisely translates the surgeon’s hand, wrist and arm movements from outside the patient into corresponding movements of an end-effector inside the patient’s body. The 3D/FlexDex platform offers an alternative to high-cost robotics in minimal access surgery by providing the visualization and wristed instrumentation for suturing found in robotic technology, but at a fraction of the cost. Importantly, this technology can be used in any OR, at any time, on a laparoscopic platform already familiar to surgeons. 3D/FlexDex can be used during many types of procedures, including but not limited to general surgery, gynecological and urologic specialties.
“Olympus is creating value and improving patient satisfaction by offering an important cost alternative to robotic surgery for physicians and hospitals,” said Randy Clark, President, Medical Systems Group, Olympus Corporation of the Americas. “The ENDOEYE FLEX 3D and the FlexDex Needle Driver combine Olympus’ advanced imaging technology with intuitive control to advance the possibilities in minimal access surgery. We are honored to have won this prestigious award.”
All nominations are reviewed by the Edison Awards Steering Committee, and the final ballot is sent to an independent judging panel. The judging panel comprises more than 3,000 professionals from the fields of product development, design, engineering, science, marketing and education, including professional organizations representing a wide variety of industries and disciplines. For more information on the 2019 Edison Awards, please visit www.edisonawards.com. Applications for the 2020 awards will open in August 2019.
# # #
About Olympus Medical Systems Group
Olympus is a global technology leader, crafting innovative optical and digital solutions in medical technologies; life sciences; industrial solutions; and cameras and audio products. Throughout our 100-year history, Olympus has focused on being true to society and making people’s lives healthier, safer and more fulfilling.
Our Medical Business works with health care professionals to combine our innovative capabilities in medical technology, therapeutic intervention, and precision manufacturing with their skills to deliver diagnostic, therapeutic and minimally invasive procedures to improve clinical outcomes, reduce overall costs and enhance quality of life for patients. For more information, visit medical.olympusamerica.com.
About FlexDex
FlexDex is an innovative medical device company that has developed a platform technology to provide surgeons with high performance and cost effective minimally invasive surgical instruments. The Company’s focus is to enable advanced minimally invasive procedures with greater precision and efficiency bringing the benefits of minimally invasive surgery to patients throughout the world. FlexDex was developed at the University of Michigan by co-founders Shorya Awtar, Sc.D., James Geiger, MD and Greg Bowles. This platform technology enables highly intuitive, one-to-one mapping of the surgeon’s arm and hand motions to the articulating instrument inside the patient’s body. The patented “Virtual Center™” of the FlexDex platform is a simple mechanical design that, we believe, will greatly enhance the capabilities of all Minimally Invasive Surgical (MIS) instruments. The FlexDex Needle Driver, the first instrument of the FlexDex platform, has been granted the CE mark and is available for sale in the United States and internationally. For more information visit https://flexdex.com